Tregs are a subset of immune cells with potent immunomodulatory functions. These functions include maintaining immune homeostasis and immune tolerance, as well as regulating proinflammatory immune responses.

CAR-Tregs are expected to utilize multiple mechanisms of immune regulation to reduce inflammation locally.
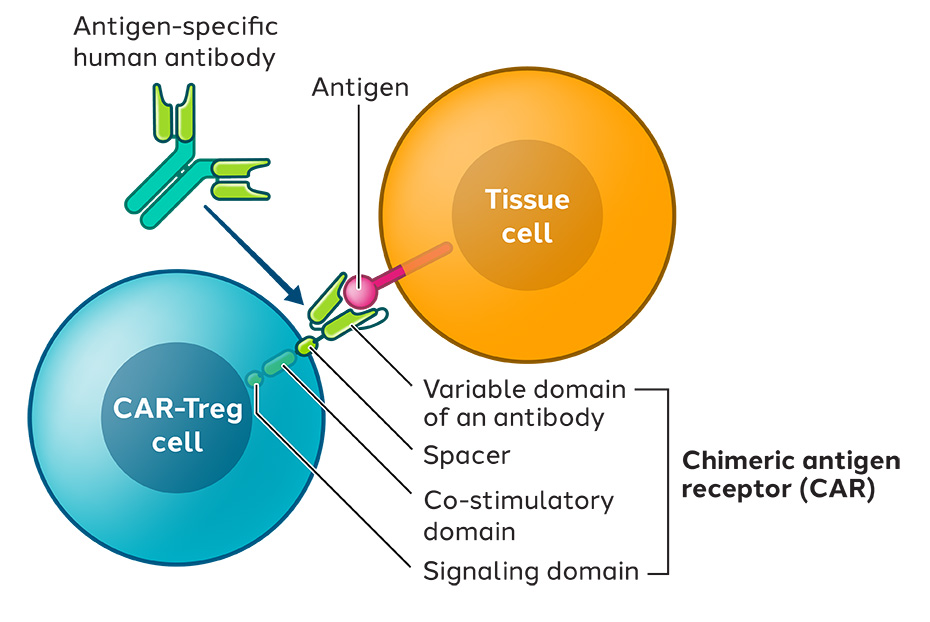
We engineer Tregs with a CAR targeted to IL23R, which is found in the GI tract and overexpressed by inflammatory cells in the gut of Crohn’s disease patients. When the CAR-Tregs are infused into the patient, they are expected to migrate to the GI tract, engage IL23R and suppress the local inflammation.

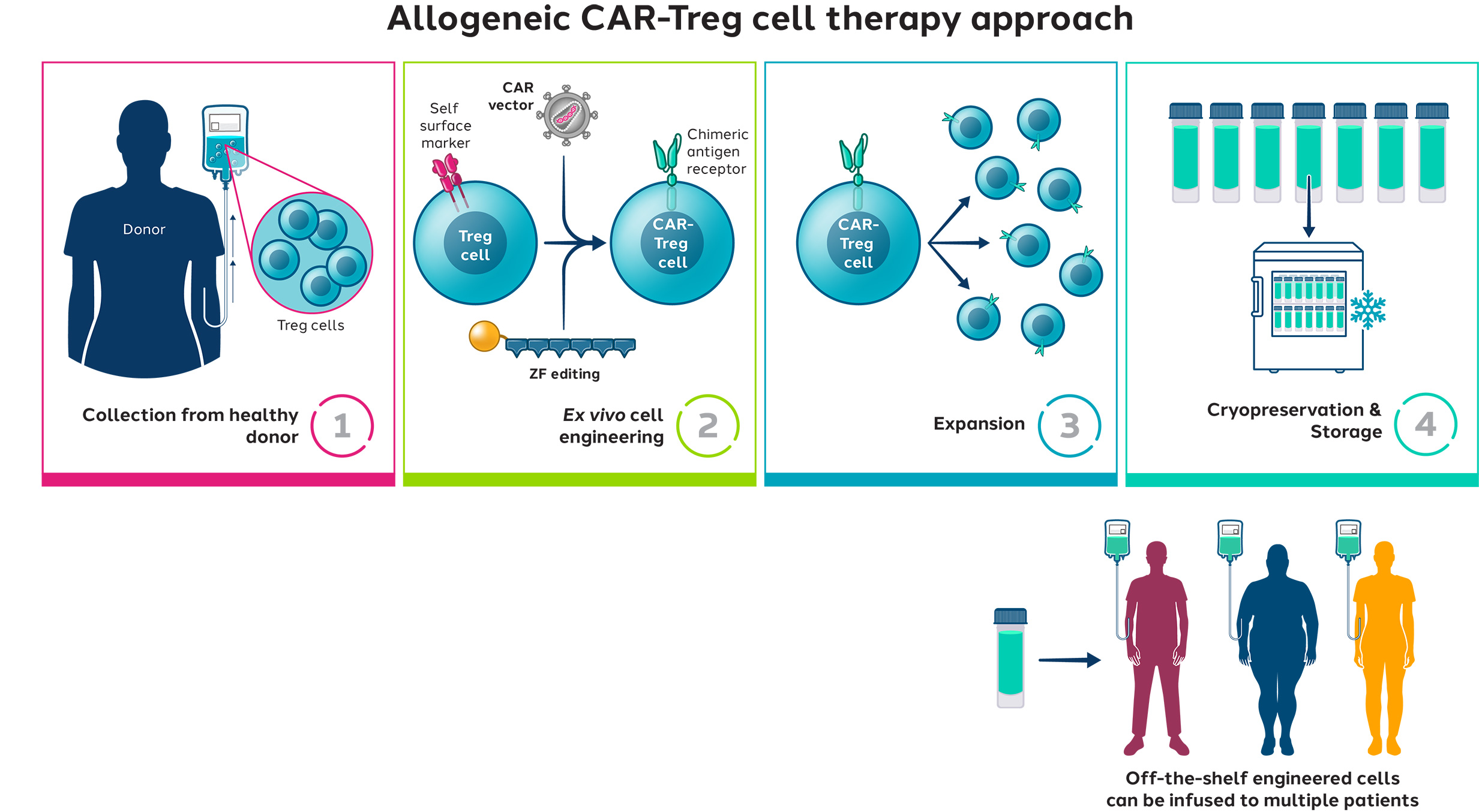
CAR-Tregs engineered by Sangamo using Zinc Finger technology are positioned to be the new frontier in cell therapy to potentially address inflammatory and immune-mediated diseases.
Sangamo’s strategy is to first evaluate first-in-human proof-of-concept in an autologous study, and then potentially move to allogeneic approaches to address scalability.