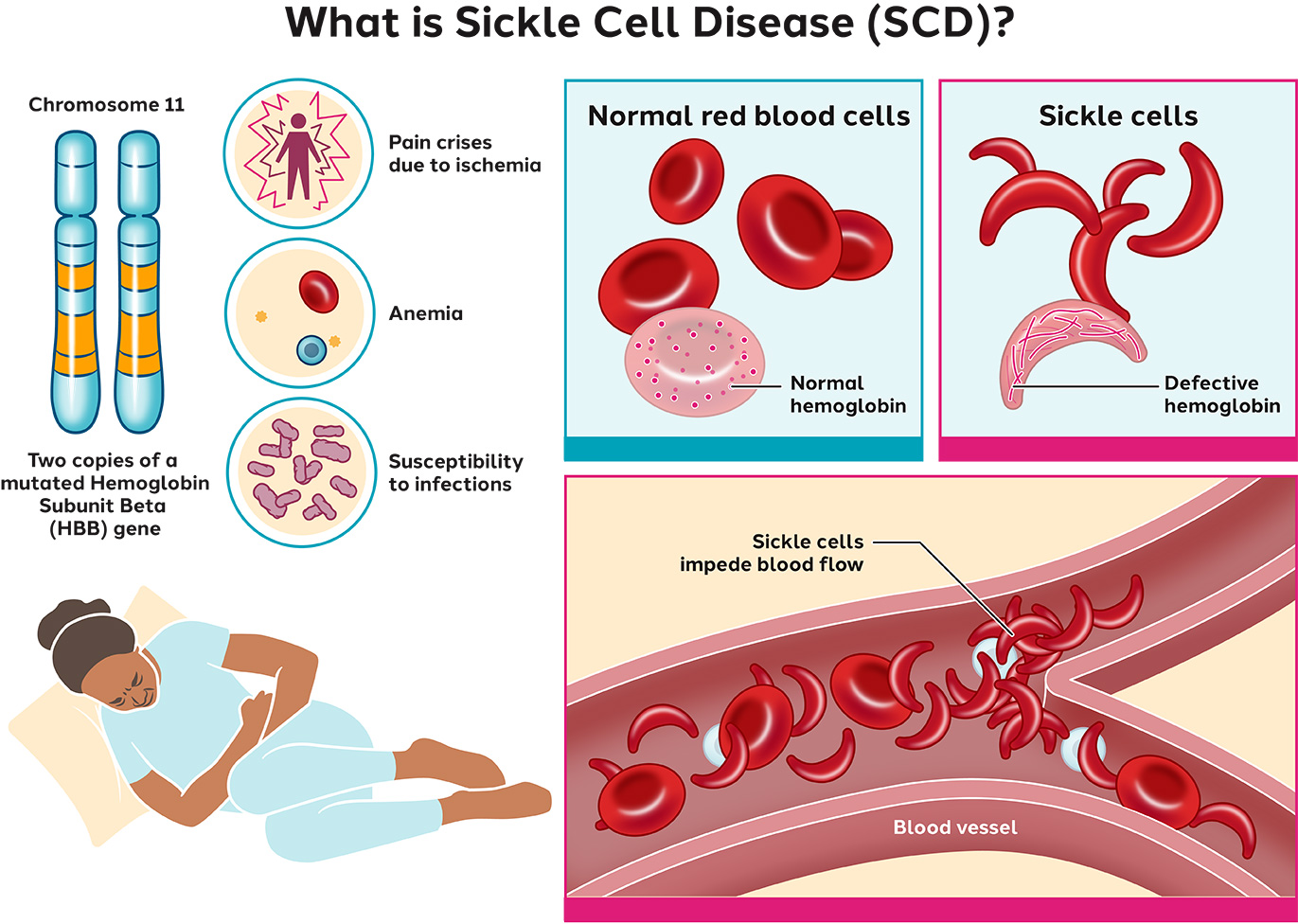
Sickle Cell Disease (SCD) is a blood disorder caused by a mutation in the hemoglobin gene, resulting in distorted red blood cells (sickle cells). These sickle cells either die early or cause blockages in small blood vessels. SCD’s main symptom is agonizing pain – either chronic or episodic – due to ischemia, a restriction in the blood flow to certain organs.

BIVV003 is a zinc finger nuclease (ZFN) gene-edited cell therapy candidate. BIVV003 is manufactured by ex vivo gene editing of a patient’s own (autologous) hematopoietic stem cells using non-viral delivery of ZFN technology targeting the BCL11a gene erythroid-specific enhancer. As this gene naturally stops the production of fetal hemoglobin (HbF) in the first few months of life, its deactivation by BIVV003 is expected to restore the production of functional HbF and relieve SCD symptoms.
PRECIZN-1 is an ongoing first-in-human, open-label, single-arm, multicenter Phase 1/2 clinical study in up to eight patients with SCD evaluating the safety and tolerability of cell therapy candidate BIVV003.
BIVV003 has received Fast Track Designation from the U.S. Food and Drug Administration (FDA) and Orphan Medicinal Product from the European Medicines Agency (EMA).