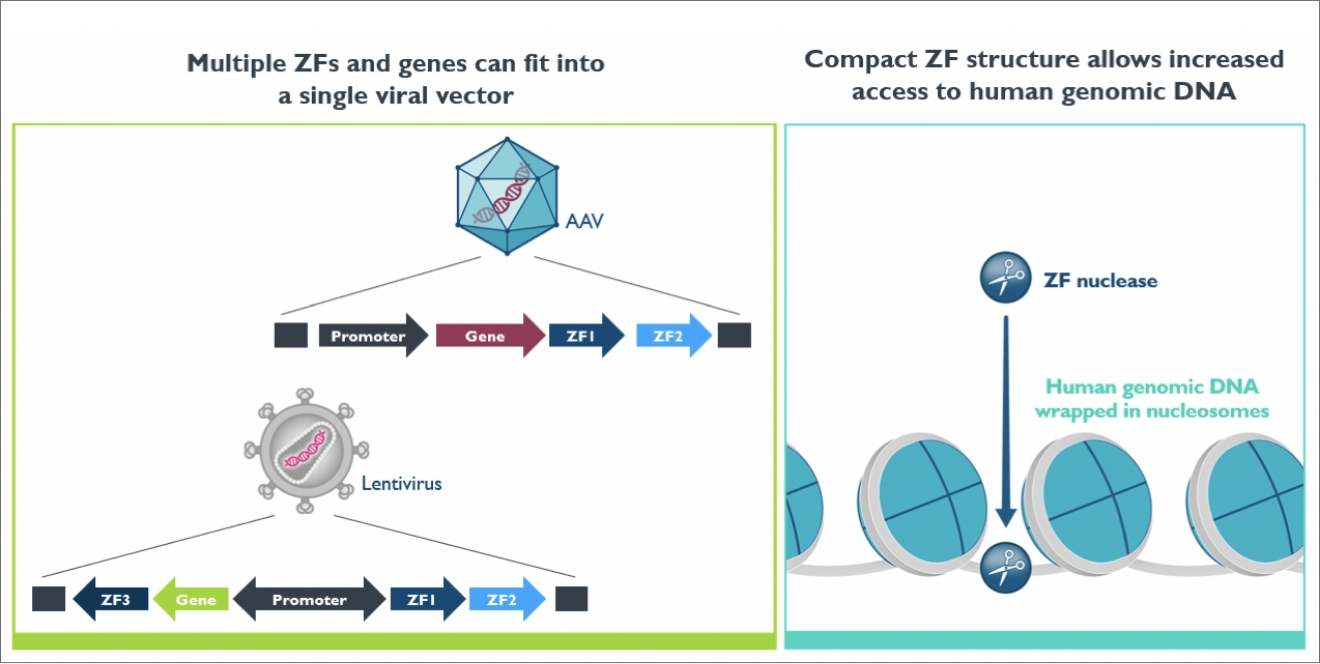
Depending on the desired therapeutic application, our zinc finger technologies may be used either on human cells outside the body (ex vivo) or directly on cells and tissues inside the body (in vivo). In both cases, zinc finger technologies need to be carried and brought into the targeted cells.
To achieve this, we use “delivery vectors”, which are vehicles used to transport genetic material. These vectors can be derived from inactivated viruses, such as adeno-associated virus (AAV) or lentivirus (LVV).
Committed to refining zinc finger technologies, our goal is to offer effective and safe therapeutic options that address the needs of patients with diverse diseases. Our considerable experience using AAVs in clinical trials supports our belief in the safety of AAVs for delivering genomic medicines. To date, more than 200 patients across our various clinical trials and therapeutic areas have been dosed with AAV therapies that have been generally well tolerated.
Zinc finger proteins are compact proteins, which not only allows greater genome accessibility, but also makes them highly compatible with viral vectors, greatly improving our ability for cellular delivery.

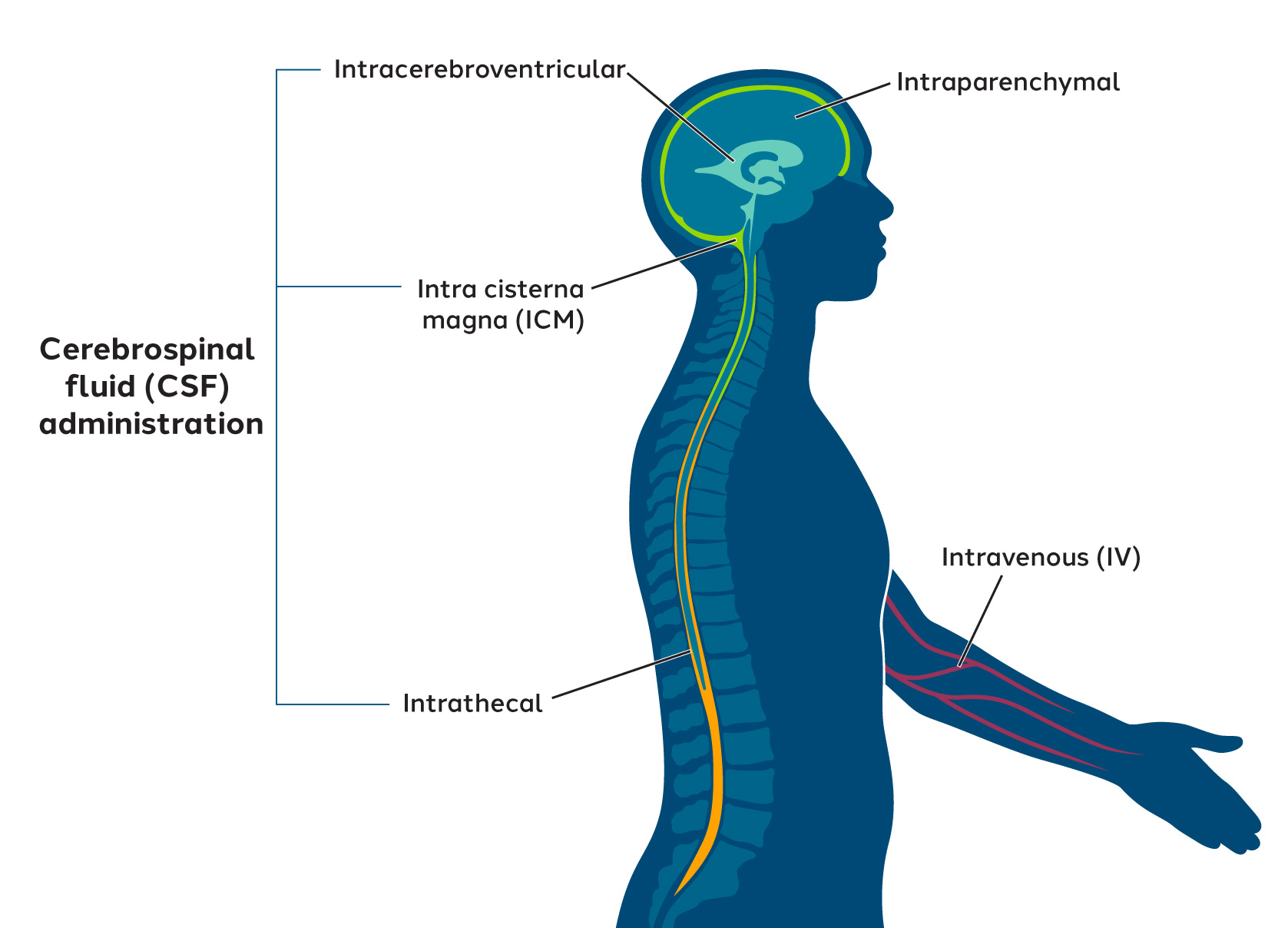
Our goal is to develop potential therapies that can effectively treat neurological disorders. However, delivering genomic medicines that overcome biological barriers to reach the nervous system presents significant obstacles.
One promising approach is to use adeno-associated viruses, or AAVs, as delivery vehicles. Conventional AAVs have limitations that can hamper their effectiveness for widespread nervous system delivery. To address these challenges, we have developed a novel AAV capsid discovery platform and are evaluating several potential routes of administration for our neurology-targeted investigational therapies.
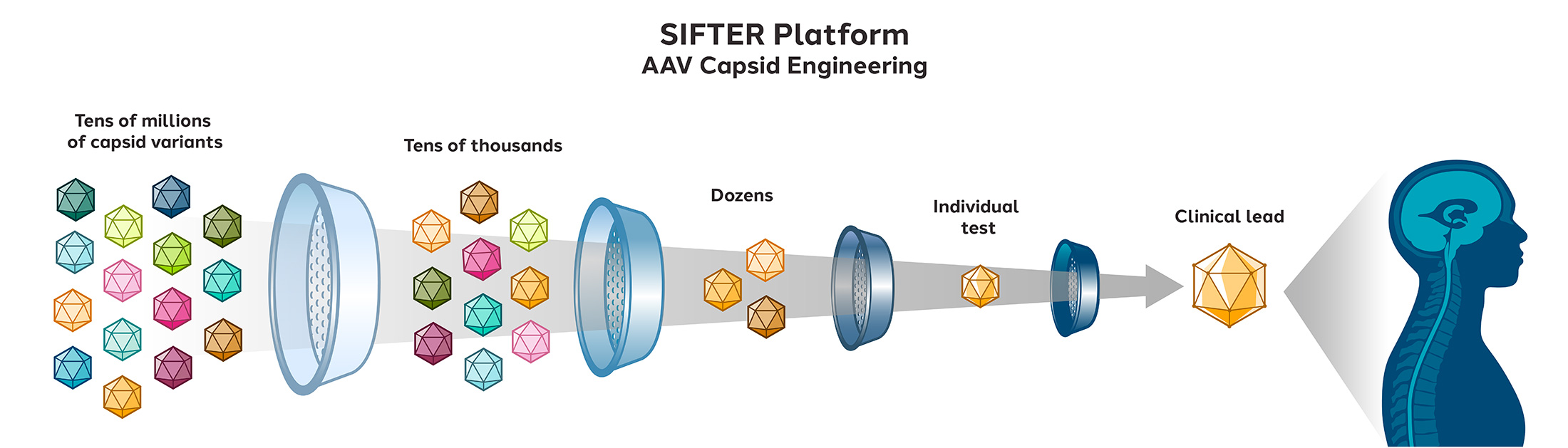
Our proprietary AAV capsid discovery platform is called SIFTER (Selecting In vivo For Transduction and Expression of RNA). This platform allows us to engineer capsids with improved nervous system transduction.
Using SIFTER, we are screening tens of millions of unique capsids to identify those that mediate superior delivery to the nervous system. We conduct successive rounds of screening to find capsids that reproducibly demonstrate a desired therapeutic profile.
This platform enabled us to identify in May 2022, new capsids, STAC-102 and STAC-103 (STAC = Sangamo Therapeutics AAV Capsid), that exhibit improved delivery to the nervous system relative to conventional AAV capsids via intrathecal administration.
In March 2024, we announced data for our proprietary AAV capsid variant, STAC-BBB, which demonstrated an ability to cross the blood-brain barrier (BBB) in non-human primates (NHPs) and mediated robust transduction, transgene expression, and targeted, potent epigenetic repression throughout the brain after intravenous administration. STAC-BBB also demonstrated industry-leading brain tropism and enrichment in NHPs, resulting in 700-fold higher transgene expression than the benchmark capsid AAV9 and outperformed all other known published neurotropic capsid variants evaluated in the preclinical study.
We believe that improved AAV capsids with higher delivery efficiency and specificity for target tissues have strong potential to create safe and effective genomic medicines to treat neurological disorders. By overcoming the obstacles to nervous system delivery, we can unlock new opportunities for potentially treating these disorders and improving patients’ lives.