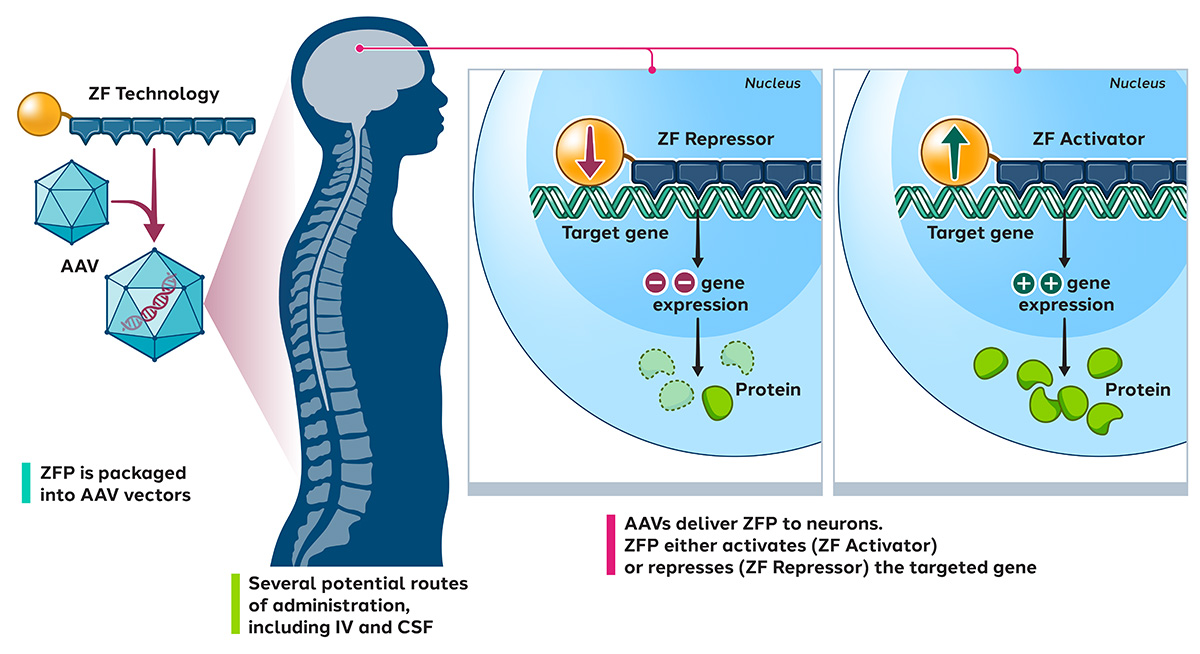
For these diseases, we design zinc finger transcriptional regulators (ZF-TRs) to modulate gene expression levels in the brain. We package these into AAV vectors designed specifically for nervous system delivery.
Our preclinical neurological targets include chronic neuropathic pain, prion disease, Huntington’s disease and ALS.

Neuropathic pain is one of the most difficult pain syndromes to manage and can be caused by a broad array of pathologies impacting the central or peripheral nervous systems, such as surgical trauma, spinal cord injury, nerve compression, neurological and infectious diseases, or metabolic and hereditary syndromes.
A significant body of evidence implicates sodium channels in mediating the pathophysiology of neuropathic pain. We are developing ST-503, a zinc finger repressor targeting the human gene SCN9A that encodes the Nav1.7 sodium channel. Developing small molecules that specifically target Nav1.7 is challenging due to the high structural similarities between different sodium channels, making it difficult to achieve selectivity and avoid off-target effects. We believe our epigenetic regulation approach is differentiated. By directly targeting the SCN9A gene, ST-503 has shown to precisely and potently reduce the expression of Nav1.7 sodium channels in sensory neurons in animal models and significantly reduce pain hypersensitivity following a single intrathecal administration. ST-503 has been well tolerated in nonhuman primates, with no off-target effects observed.
Following FDA clearance of the IND in November 2024, we are actively preparing for a Phase 1/2 study to assess the safety, tolerability and preliminary efficacy of a one-time dose of ST-503, administered intrathecally to patients with intractable pain due to small fiber neuropathy (SFN), a type of chronic neuropathic pain.
SFN has an estimated prevalence of 53 people out of every 100,000 in the U.S., and more broadly, peripheral neuropathies are estimated to affect nearly 40 million Americans. Antidepressants, anticonvulsants, opioids and topical therapies are potential treatment options, although no long-lasting or curative therapies are currently available for SFN patients, leading to a high unmet medical need for this patient population.
ST-503 is not intended for sporadic or acute pain, but for chronic, intractable pain that completely dominates and often destroys the lives of patients over many years.
Latest preclinical data from chronic neuropathic pain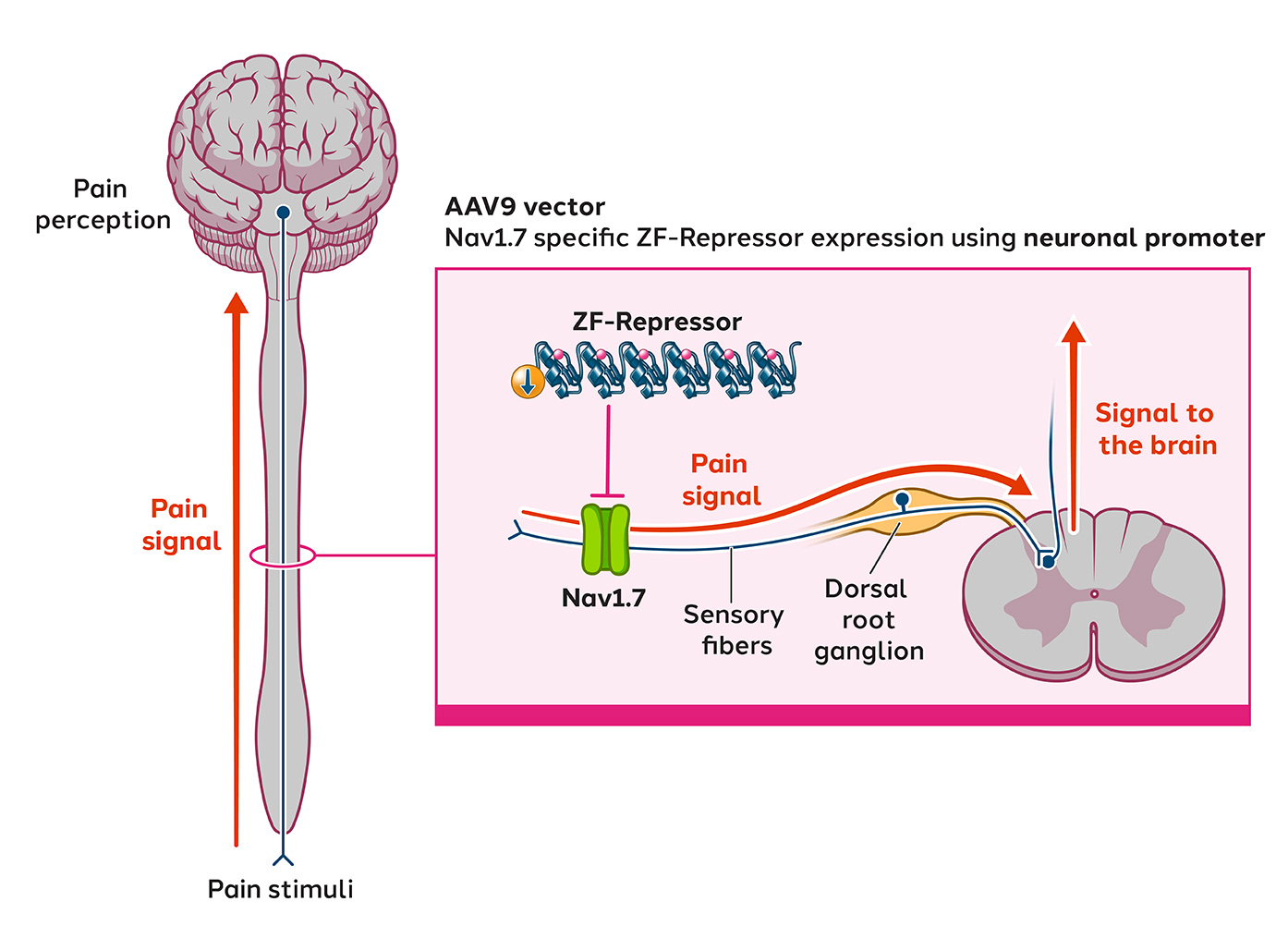
Prion disease is a fatal and incurable neurodegenerative disease caused by the misfolding of the prion protein encoded by the PRNP gene. To address prion disease, we are developing zinc finger repressors targeting the PRNP gene.
Our aim is to remove a significant portion of prion protein from neurons to protect them from the toxicity of the misfolded prion protein, potentially preventing the spread and propagation of misfolded prion, and potentially slowing or halting neurodegeneration and disease progression.
Our compelling preclinical data, presented at ASGCT 2025 and the Prion 2024 conference, showed the powerful combination of our potent zinc finger repressor—and our proprietary STAC-BBB capsid as a promising potential one-time intravenous treatment for prion disease.
Latest preclinical data from our Prion program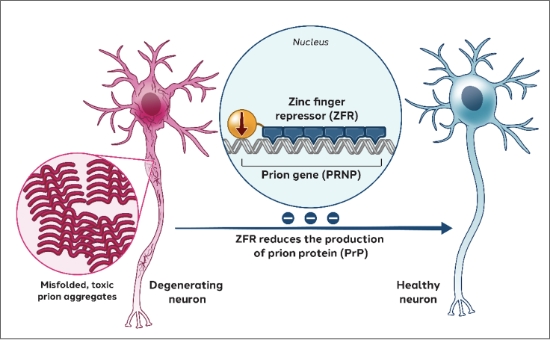
Tauopathies, such as Alzheimer’s disease, are a group of neurodegenerative disorders characterized by the accumulation of abnormal tau protein, which leads to the destabilization of microtubules in neurons and damages neuronal communication.
In March 2021, we published preclinical data in Science Advances, showing that tau-targeted ZFRs selectively reduced tau messenger RNA and proteins by 50% to 80% out to 11 months without detectable off-target events.
In August 2024 Sangamo announced a global epigenetic regulation and capsid delivery license agreement with Genentech to develop novel genomic medicines for neurodegenerative diseases, of which Tauopathies was one of the licensed indications.
Preclinical data for Tau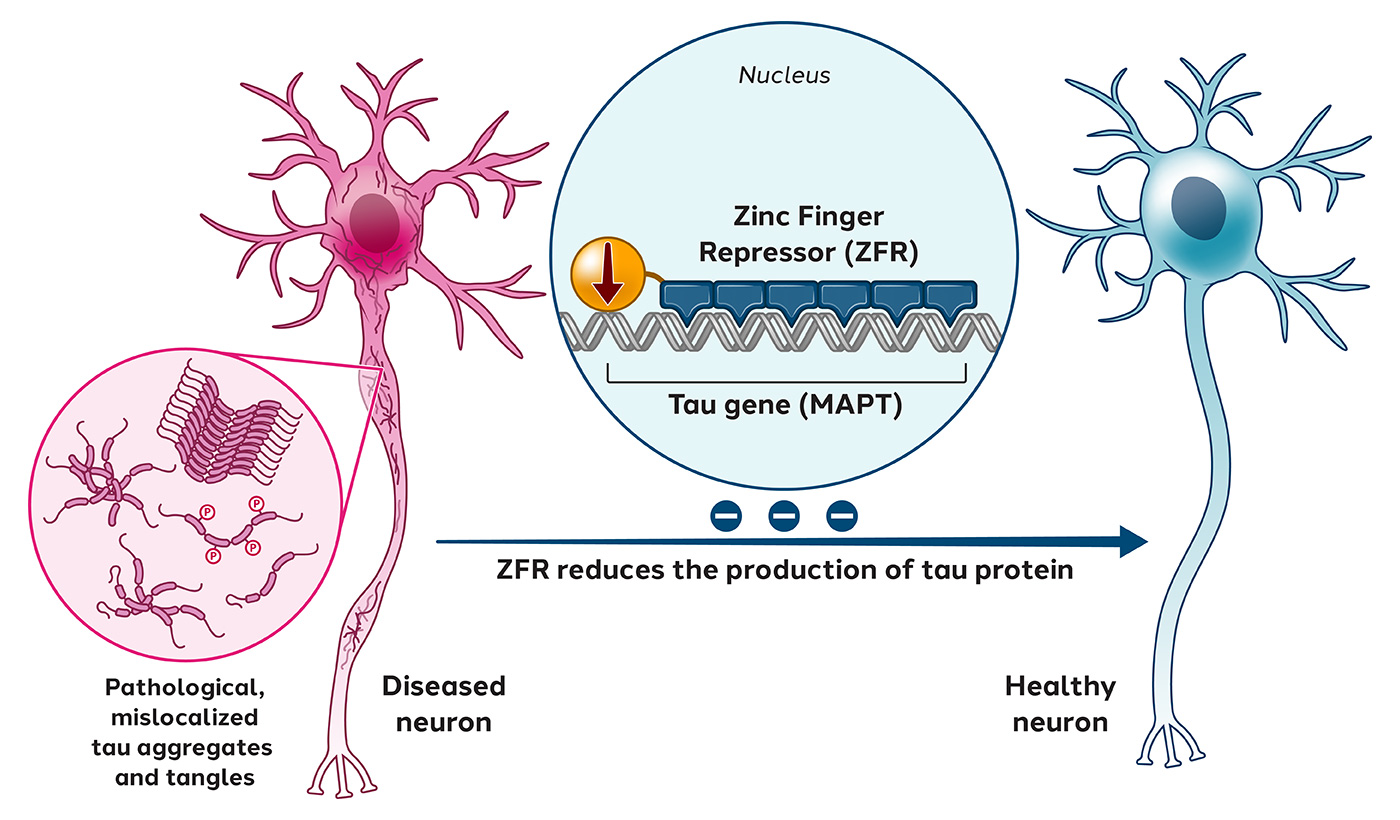
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder that primarily affects motor neurons responsible for controlling voluntary muscle movement. As the disease progresses, it leads to muscle weakness, atrophy, paralysis, and eventually respiratory failure and death.
ALS has been linked to mutations in the C9ORF72 gene, leading to abnormal protein aggregates and subsequent motor neuron degeneration. To address this disease, in partnership with Alexion (previously partnered with Pfizer), we are developing zinc finger repressors targeting the pathogenic allele of the C9ORF72 gene while preserving expression of the healthy allele.
Latest preclinical data from our ALS program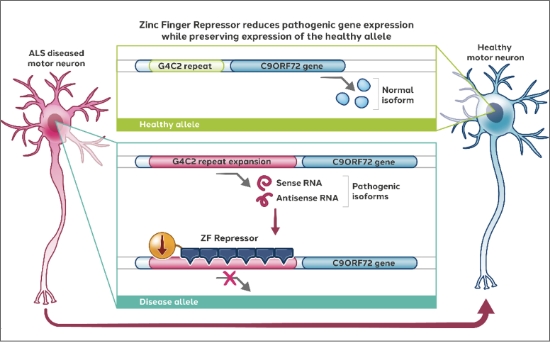
Synucleinopathies, such as Parkinson’s disease, are a class of neurodegenerative disorders marked by the abnormal aggregation of alpha-synuclein within nerve cells, causing progressive neurological decline. Our lead product candidate for the treatment of synucleinopathies, ST-502, utilizes a zinc finger repressor targeting the alpha-synuclein gene, SNCA.
In 2021, we presented preclinical data at the 15th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD) and at the ASGCT Annual Meeting, showing that alpha-synuclein-targeted zinc finger repressors could significantly repress human alpha-synuclein and were well tolerated in vivo.
Preclinical data for alpha-synucleinOur ZF-activators are able to increase the expression of a gene, which is required to potentially address neurodevelopmental disorders such as autism spectrum disorder and intellectual disability, for which limited therapeutic treatments currently exist. Data presented at ESGCT 2023 showed how ZF-As can be designed to restore normal gene and protein expression of SCN2A in vitro and in vivo. This was accompanied by a poster presentation demonstrating Shank3 gene activation, mediated by ZF-As as a potential therapeutic approach for Phelan-McDermid syndrome.
Latest preclinical data from neurodevelopmental disorder programsIn partnership with Takeda, we are developing potential preclinical genome engineering product candidates to treat Huntington’s Disease. These treatments utilize zinc finger repressors designed to differentially downregulate the mutated disease-causing huntingtin gene (HTT gene) while preserving the expression of the normal version of the gene.
Latest preclinical data from our Huntington’s disease program